Hydrolab – Membrane techniques

Membrane techniques allow for the separation of impurities with particle and particle dimensions at the molecular or ionic level. These are processes new, and their rapid development has been observed in recent years. Progress in research, in the development of membrane techniques make their application in environmental protection real technically and economically advantageous. Membrane separation processes and reactors Membrane today are techniques with a wide range of applications. Operation integration membrane with traditional technologies or designing new cycles based on membrane techniques, is becoming an attractive field of research engineering.
Application
Currently, more and more polymer and inorganic membranes, with a large selectivity and efficiency and a high degree of thermal, chemical and chemical resistance mechanical, are used for desalination of sea water, wastewater treatment, recovering valuable components from wastewater, as well as separating mixtures of compounds organic.
Generally, each membrane is a filter and, as in normal filtration, at least one of the components of the separated mixture can pass through unhindered membrane, while others are retained by it.
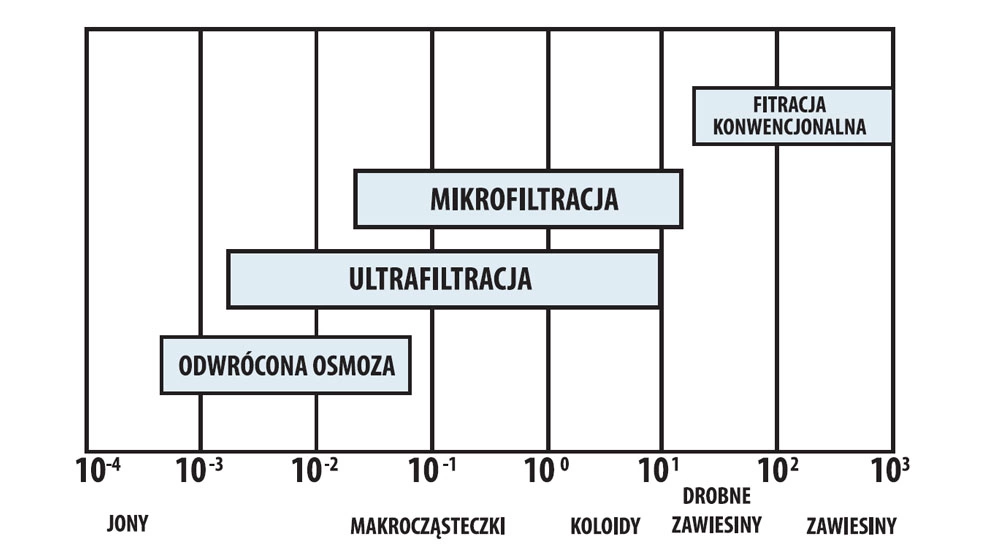
Fig. 1. Comparison of pressure methods of membrane processes in terms of retention particles.
2. Division of pressure membrane techniques
a) Microfiltration – MF
The term microfiltration refers to a process in which particles with diameters of 10-50 μm are separated from the solvent and low molecular weight components of the solution. Mechanism separation is based on a sieve mechanism and occurs only by particle diameters. Microfiltration synthetic microporous membranes are generally used pore diameter from 10 μm to 50 μm. This process allows the separation of aqueous solutions sugars, salts and also some proteins as a filtrate, leaving it in concentrate the finest particles and colloids. The driving force of the process is the pressure difference of from 0.01 to 0.1 MPa. It is generally accepted that microfiltration is used in industry and in the laboratory for the removal, concentration and purification of particles (particles) in diameter greater than 0.1 μm.
Microfiltration membranes can be prepared from organic polymers and inorganic materials (ceramics, metals, glass) using the following techniques production:
– modeling and sintering,
– stretching polymer films,
– bombardment of polymer films in the nuclear reactor,
– phase inversion.
Polymer membranes are made of both hydrophobic polymers and and hydrophilic. Ceramic membranes are prepared mainly from alumina and dioxide zirconium. For the production of inorganic membranes are used: glass, metals (palladium, tungsten) and sintered materials with carbon.
b) Ultrafiltration – UF
Ultrafiltration is a relatively low-pressure utilizing process porous symmetrical or asymmetrical membranes with pore diameters from 1 μm to 10 μm, allowing flow through the membrane e.g. sugars, salts, water, separating proteins and larger particles. There is no osmotic back pressure in the ultrafiltration process, and the separation is based, as in microfiltration, on the physical screening of particles substances dissolved or colloidal through a membrane with suitable porosity.
Diffusion processes play a small role in the separation mechanism. Applied pressure generally do not exceed 1 MPa. Unlike microfiltration, in the ultrafiltration process asymmetric membranes are used. Ultrafiltration membranes are also the basis the so-called skeleton bottom bracket with composite membranes used in others membrane techniques such as reverse osmosis, pervaporation and gas separation. Ultrafiltration is primarily used for removing, concentrating and purifying substances macromolecular and colloidal.
c) Nanofiltration – NF
Membranes that allow some ions to flow are used in nanofiltration, especially monovalent e.g. sodium or potassium. Nanofiltration is a process relatively new, which became possible to implement after developing methods production of suitable membranes. The pressures used for nanofiltration vary within 1 to 3 MPa. Nanofiltration is usually used when it needs to be removed from a solution e.g. proteins, sugars and other large particles, leaving salts in the filtrate. So far, nanofiltration has been successfully used on a technical scale in underground and surface water treatment processes, in the softening process water.
d) Reverse Osmosis – RO (ang. Reverse Osmosis)
Reverse osmosis is used to separate small molecules (salts inorganic, low molecular weight organic compounds) from solvent. It is necessary using higher transmembrane pressures than in the case of ultra and microfiltration, because low molecular weight compounds have higher pressures osmotic. These pressures depend more on the concentration than for the solutions macromolecular compounds.
Natural osmosis
The phenomenon of reverse osmosis is based on the phenomenon of natural osmosis. In a system where the membrane separates the solution from the solvent or two solutions at different concentrations, spontaneous penetration of the solvent through the membrane occurs towards a more concentrated solution. External pressure balancing the flow osmotic is called osmotic pressure, and is characteristic of the given solution.
Reverse osmosis
If the solution side creates a hydrostatic pressure that exceeds osmotic pressure, the solvent will permeate from the more concentrated solution to diluted, and thus in the opposite direction than in the process of natural osmosis. Reverse osmosis was proposed for this process. It is used in parallel sometimes name hyperfiltration. Reverse osmosis allows the solvent (water) to be separated from solutes, even with relatively low molecular weight, e.g. salts and sugars. The separation mechanism is diffusive. Working pressures used in the reverse osmosis process due to the high value of osmotic pressures of the separated solutions are high and range from 1 to 10 MPa.
Application
Reverse osmosis was first used in 1953 for desalination sea water. It was introduced to industry only in the sixties after Loeb and Sourirajan developed an industrial scale manufacturing technology high-performance, yet selective, asymmetrical membranes. It is a process separation of low molecular weight components (M <300). Diameters of separated particles and molecules can range from a few to a dozen or so angstroms (Ǻ). Particles and particles retained by the membrane lead to an increase in concentration on this side of the membrane, making with in turn, causes an increase in osmotic pressure, which reduces the driving force of the process.
The filtrate (permeate) flow is possible when the external pressure (Δp) exceeds osmotic pressure (π).
π = C·RG·T
Depending on the concentration of the solution on both sides of the membrane, the range used pressure ranges from 0.3 – 10 MPa. Unlike a traditional filter, reverse osmosis can separate solution components to a molecular size range, making it competitive with other water purification methods. It is possible to combine membrane units with classic chemical engineering processes, e.g. ion exchange, distillation, crystallization.
The essence of reverse osmosis is shown in Figure 2:
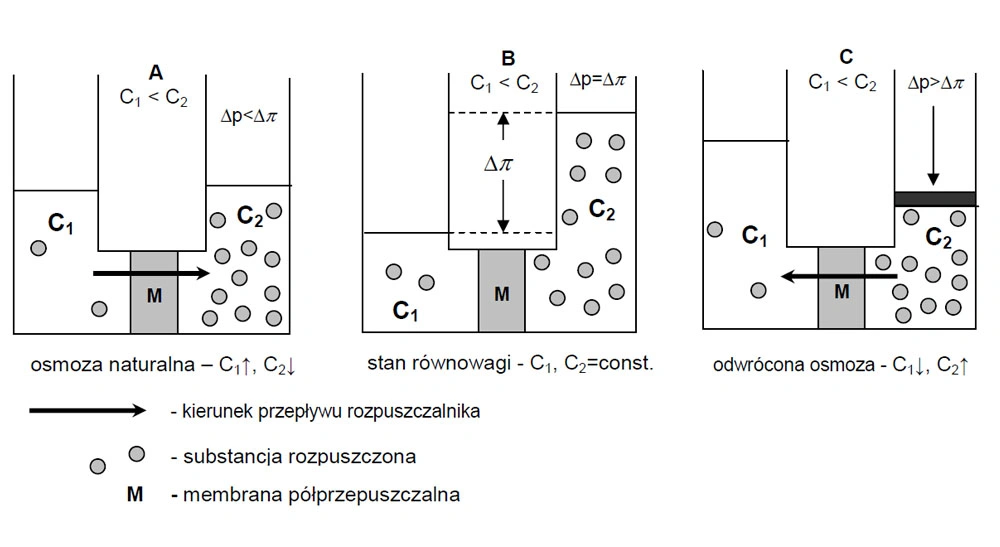
A – natural osmosis
When a perfectly semi-permeable membrane divides two solutions of different concentrations (C1, C2), a chemical potential difference Δμ arises on both sides of the membrane. There is a spontaneous flow of solvent from the lower concentration solution to the solution with higher concentration (C1↑, C2↓), (Δp<Δπ)
B – balance
At equilibrium between these solutions, a pressure difference of equal is established osmotic pressure difference of both solutions (C1, C2=const.), (Δp=Δπ).
C – reverse osmosis
If a higher pressure Δp is applied to a solution with a higher concentration than water, it will flow into the solution with a lower concentration, i.e. in the opposite direction of the osmotic stream. Reverse osmosis then takes place, leading to concentration of this solution and dilution of the solution on the opposite side membranes (C1↓, C2↑), (Δp>Δπ). The driving force of this process is the pressure difference equal to: Δp – Δπ.
3. The concept of a membrane
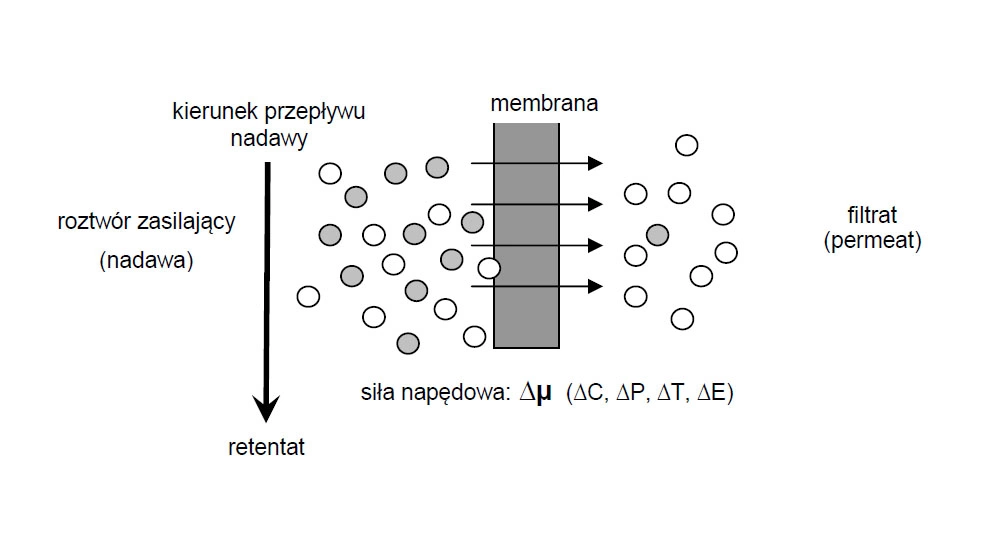
A common feature of all membrane techniques is that the separation process is due to the presence of a membrane (Fig. 2.). Under the concept of membranes, according to Recommended by Membrane company, we understand the phase separating two other phases, which acts as a passive or active barrier for mass transport between them. According to another, more general membrane, it is internationally permitting controlled transport of one or more components from a set of solid, liquid or gas. Flow direction (kierunek), feed solution (roztwór zasilający), retentate, driving force (siła napędowa), filtrate (filtrat).
4.Basic parameters of membrane processes
Membrane techniques, despite their short history of use, occupy high position among currently popular separation methods. Efficiency of using membrane is determined using one of two parameters: retention rate possible or selectivity.
A common feature of all semi-permeable membranes suppliers in processes permeation is varied mass transport speed, which depends on the type and values of driving forces covered on the basis of phase separated components and from general and chemical properties of the membrane.
flux rate
Solution volume flow jp [dm3/min*m3] otherwise flux rate is a measure of the intensity of the membrane process. It is determined by volume passed through the membrane used under the influence of motive force by use membrane usable area and available time.
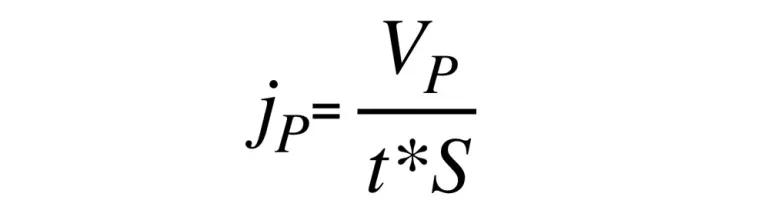
where:
VP – volume of solution, m3,
t – time, s or d, s lub d,
S – membrane surface, m2.
The filtration rate [jP] and the amount of solute passing through can be related equation in which the membrane surface and working time are constant:
ds=jP*CsP
where:
ds – solute flow, mol, mol/(m2*s),
CsP – concentration of solute in permeate, mol/m3
The effect of separation of the components flowing through the membrane results from differentiation of their transport speed and different solubility in the membrane material.
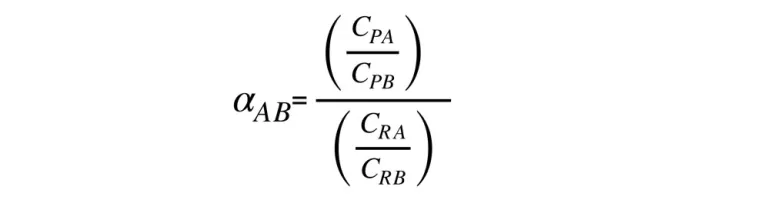
Separation selectivity α ABof two components A and B transported through the membrane expresses the separation coefficient defined by the ratio of the concentration ratio (A) and (B) in permeate and retentate:
where:
CPA, CPB – concentrations of component A and B in the permeate,mol/m3,
CRA, CRB – concentrations of component A and B in the retentate, mol/m3
The separation effect can also be determined by the retention factor R, i.e. degree of retention (salt-rejection):
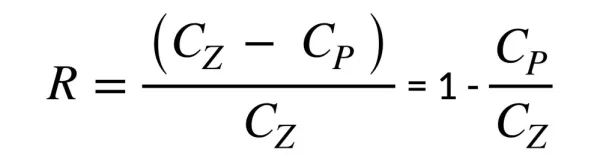
where:
CZ – concentration of solute in the separated solution, mol/m3,
CP – concentration of solute in filtrate, mol/m3.
To assess the effectiveness of the permeation process is used so-called: degree Y conversion (recovery), defined as follows:
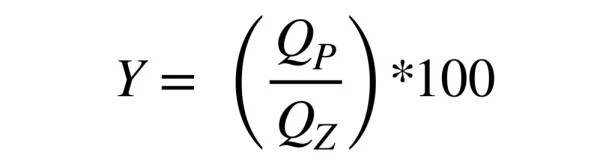
where:
QP – permeate flow rate, m3/s,
QZ – feed solution flow rate, m3/s
5. Reverse osmosis
The mechanism of mass transport through the membrane in the RO proces
The mechanism of separation in reverse osmosis describes the dissolution – diffusion model. This model assumes that the flow of specific components through compact membranes polymeric is determined by their dissolution in the polymer and diffusion. The model ignores the impact between the membrane polymer and the diffusing component. Ingredients diffuse through membrane under the influence of a “thermodynamic stimulus”, i.e. a negative gradient chemical potential of this component.
Reverse osmosis, however, is definitely different from other techniques of this type, such as ultra- and microfiltration. In MF and UF processes, the basis of separation is the sieve effect. Meanwhile, in RO this effect practically does not occur.
Membranes in the RO process
In the process of reverse osmosis, asymmetric membranes constructed of are used one polymer and composite membranes. The thickness of the active layer is usually £ 1mm, with permeability determined by the active layer. For the production RO membranes are usually used cellulose esters, primarily cellulose di- and triacetate, because they have hydrophilic properties. Cellulose acetate is low thermal and microbiological resistance and is hydrolysed at low and high pH solution. Another material for making membranes are aromatic polyamides, which are not very resistant to free chlorine.
The new generation of RO membranes are composite membranes in which a layer active and bottom bracket are made of different polymers. The bottom bracket is usually ordinary ultrafiltration (polysulfone) membrane. Active layer is made of polymers such as: polyimides, polybenzimidazole, polybenzimidazolate, polyamide hydrazine.
The polymer from which the membrane is made and the epidermal layer of the membrane for RO, in order to ensure high selectivity:
– should be in glassy state,
– it should be mechanically strong,
– the molar mass of the polymer should be high enough and the molar mass dispersion as least,
– should have high hydrolytic resistance (i.e. hydrolysis resistance), so that the membrane’s durability is 3-5 years,
– should not be biodegradable, – it should be resistant to chlorine and other oxidants.
The use of reverse osmosis
From the range of available opportunities to use reverse osmosis, applications specifically defined by the development of the above specified directions, the most relevant apply Include:
– desalination of saltwater and brackish waters,
– cover mine waters,
– obtaining rinsing water in photography to recover silver,
– recovery of soda from drainage water of a coal mine,
– wastewater treatment from textile dyeing plants,
– include pulp washing,
– include water from landfills,
– water softening,
– embracing the sulfite liquor,
– taking into account the waste water used solvents.
