Call 042-300 91 30 or email info@labteamet.com
Centrifugering är en teknik som använder g-kraft, för att separera olika typer av mixer. Centrifugen är en apparat som oftast har en rotor, som roterar runt en fast axel.
Centrifugen fungerar enligt principen för sedimentation. Under påverkan av g-kraften separeras provet, beroende på beståndsdelarnas densitet. Det finns flera typer av kända separationsprocesser. För att nämna några så finns det ultrafiltrering, fasseperation och pelleting (se bild 1)
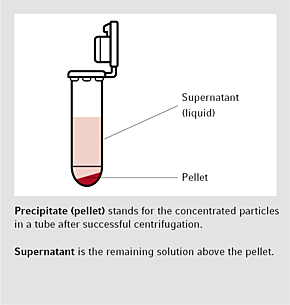
Bild 1 Bildande av pellets efter centrifugering
Pelleting är den vanligaste applikationen. I denna form av centrifugering bildar partiklarna en pellets, i botten av ditt centrifugrör och vätskan som blir kvar, kallas supernatant. Under seperationsfasen, så omvandlas kemikalierna, från ett matrix/vattenlikt media till en lösning. Under ultrafiltration, renas och separeras makromolekyler, med hjälp av ett filter. separated. Isopyknisk centrifugering, fungerar genom att det bildas en gradering avseende densitetis genom jämviktssedimentering. Protokoll för centrifugering brukar ta hänsyn till rcf (relative centrifugal force) och graden av acceleration i multipla g (g-kraft). Att använda rpm (revolutions per minutes) blir väldigt oprecist.
2). Skillnaden mellan rpm och rcf, är betydande, då två rotorer med olika diametrar, men vid samma rpm, kommer ge upphov till två olika rcf. Nedan kommer förklaringen.
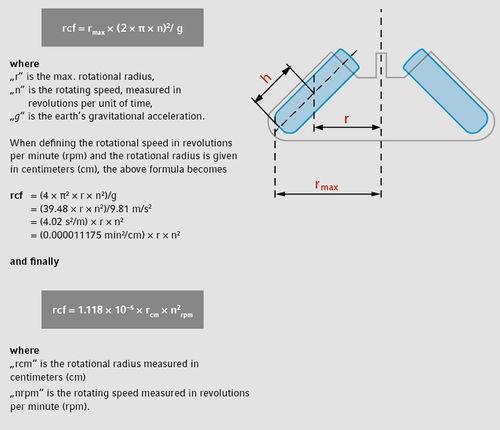
Bild 2 Uträknande av rcf
Eftersom rörelsen på rotorn är cirkulär, så räknas accelerationskraften (× g) ut, som en produkt av radien och kvadraten på hastighetsvinkeln. Resultatet av denna ekvation är det som historiskt sätt kallats rcf “relative centrifugal force”.
Exempel (beräkning enligt bild 2)
| Rotor A | Rotor B | |
|---|---|---|
| Hastighet | 14,000 rpm | 14,000 rpm |
| Radie | 5.98 cm | 9.50 cm |
| Gravitation | 13,100 × g | 20,817 × g |
Båda centrifugerna kan centrifugera med en rotor, med rör som innehåller 1.5/ 2 mL, vid samma hastighet (14,000 rpm), men accelerationen är olika 13,100 × g kontra 20,817 × g. Detta ger upphov till olika resultat. För att underlätta, har många centrifuger en knapp som direkt kan konvertera rpm till rcf och vise versa. Om inte din centrifug har denna automatiska knapp, går det att räkna ut, neligt formeln ovan. K-faktorn är en parameter för distansen av sedimentation i ett rör. Denna faktor kallas också upprensningsfaktorn och representerar den relativa efektiviteten av pelletsbildning vid maximal rotationshastighet, för ett centrifugsystem. Vanligtvis används k-faktorn, som ett värde för att estimera tid (i timmar), för att ett visst prov, med en viss sedimentationskoefficient, skall sedimentera. Sedimentationskoefficienten mäts i S (Svedberg).
En liten k-faktor, representerar en snabbare seperation. Värdet på k-faktorn är primärt definierat av rotorns diameter. I jämförelse med rpm/rcf, har k-faktorn fått en mindre betydelse, vid dagens centrifugeringsprocesser. För ultracentrifugering är dock k-faktorn fortfarande av mycket stort värde.
Det finns två typer av centrifuger, golvmodeller eller bänkmodeller.
Golvmodellerna kan oftast centrifugera vid mycket höga g-tal och har stor kapacitet. Dock tar dessa centrifugera ganska stor golvyta. Dessa centrifuger används bland annat vid ultracentrifugering.
När det gäller bänkcentrifugerna, finns dessa i två varianter, mikrocentrifuger eller multifunktionscentrifuger. Mikrocentrifugerna används oftast till rör (mikrotiterrör) med liten volym. Dessa centrifuger är små, smidiga, men har en begränsad kapacitet och möjlig g-kraft. När det gäller multifunktionscentrifugerna har dessa ett större användningsområde och beroende på vilken rotor och vilka adaptrar, du använder, kan du centrifugera allt från rör till plattor.
Om du följer ett givet protokoll, se till att du använder samma rotor, samma hastighet, samma temperatur och samma centrifugeringstid. Om du vill ha en så optimal centrifugering som möjligt tänk på dessa parametrar:
De två vanligaste rotorerna är fastvinkel eller swing-bucket. Det finns dock andra varianter på rotorer, som t.ex. trumrotorer, och konstantflöderotorer.
Den uppenbara fördelen, är att rotorn saknar rörliga delar. Detta resulterar i att rotorns metall inte utsätts för så mycket slitage, man kan nå högre g-krafter och således korta ned den nödvändiga centrifugeringstiden. Den enda nackdelen är att denna rotor har begränsad kapacitet. Positionen, på den pellets som bildas, beror mycket på lutningen på röret. Oftast är lutningen 45°. Ju större lutning på röret, desto tightare blir pelletsen. Små rotorer ger upphov till pellets, som då får en större utspridningsyta.
Dessa rotorerna är mer flexibla och beroende på vilka insatser du använder, kan du centrifugera allt från rör till plattor. De rörliga delerna i denna rotor gör att det blir ett högre slitage, i jämförelse med en fastvinkelrotor. Eftersom slitaget blir större, kan man inte använda lika höga g-krafter och centrifugeringen tar således längre tid. Baserat på swing-bucket principen hamnar pelletsen i botten på röret.

Rotorerna snurrar med en väldig hastighet och orsakar extrema krafter. Med detta i åtanke är det väldigt viktigt att man balanserar proverna, vid körning. Extra viktigt är det när inte centrifugen är helt full av prover, då måste man verkligen tänka på hur proverna placeras, för att uppnå balans.
Om du placerar dina prover inkorrekt och ger upphov till obalans kan detta ge skador på rotorn. Obalansen kan också ge upphov till kraftiga vibrationer, som kan skada hela centrifugen. I värsta fall kan mycket stor obalans leda till att hela rotorn och centrifugen förstörs. De flesta av våra centrifuger har obalansbrytare som bryter så fort den känner av för stor obalans, det är fortfarande dock mycket viktigt att alltid balansera sina prover korrekt vid körning med en centrifug, detta ökar även centrifugens livstid.
Många centrifuger har en inbyggd kontroll av obalans (via inbyggda sensorer), som gör att centrifugen bromsar eller helt stänger av sig, om obalans förekommer. Att tänka på är att mindre modeller av centrifuger, som inte kommer upp i höga hastigheter, oftast inte har inbyggda sensorer. På dessa centrifuger märker du obalansen genom ökade vibrationer, vid centrifugering.
Om centrifugen börjar vibrera intensivt, stäng genast av den, då är det obalans. Små vibrationer är normalt vid centrifugering, medan kraftiga vibrationer kan ge upphov till skador/allvarliga skador. När du väl har stoppat din centrifug, se om proverna var obalanserade. Uppstår problem under centrifugering, trots att dina prover är balanserade, kontakta genast LabTeamet, så kommer vi ut till dig och hjälper dig och utför service och kontroll.
Automatisk igenkänning av rotorn
Många centrifuger, speciellt bänkmodeller som kan användas på flera olika sätt, har automatisk igenkänning av rotorn. Du väljer vilket RCF (g-tal) du ska köra och funktionen känner av den nyinsatta rotorn, baserat på rotor som den känt av räknar den automatiskt ut rätt rpm baserat på det RCF (G-tal) du valt att köra på. På de mindre centrifugmodellerna finns inte denna egenskap, utan där är alla rotorer anpassade efter att kunna rotera efter centrifugmodellens givna maxhastigheter.
Detta är en liten guide och inte en komplett instruktion, vi hänvisar alltid till manualen för respektive utrustning för komplett info som gäller för respektive utrustning och modell.

LEASING / RENT / INSTALLMENT
Our collaboration with Svea means that you can quickly and easily get a proposal for financing that suits your needs Financing up to 100%. You do not have to tie up your capital. Read more about lease and insurance.
Invest now in the right equipment, to ensure your competitiveness, capacity and access to vital equipment Spread the cost over the time the equipment is used. The equipment as collateral for the financing.










STOCKHOLM
LabTeam Scandinavia AB
Finlandsgatan 36
164 74 Kista
Sweden
GOTHENBURG
LabTeam Scandinavia AB
Stora Åvägen 21
436 34 Askim/Gothenburg
Sweden

LEASING / RENTAL / INSTALLMENT
Our collaboration with Svea means that you quickly and easily get proposals for financing that suits your needs Financing up to 100%. You do not have to tie up your capital. Read more about leasing and all-risk insurance.
SIGN UP FOR OUR NEWSLETTER
Your email (mandatory)
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies allows us to process data such as browsing behavior or unique IDs on this website. Not consenting or withdrawing consent may adversely affect certain features and functions.
This request is only for this product. If you want to make requests for several products at the same time, please use the “Request (multiple products)” button on the respective product page instead and collect your products under “My products” before sending your request.