Ring 042-300 91 30 eller maila info@labteamet.com
Centrifugation is a technique that helps to separate mixtures by applying centrifugal force. A centrifuge is a device, generally driven by an electric motor, that puts an object, e.g., a rotor, in a rotational movement around a fixed axis.
A centrifuge works by using the principle of sedimentation: Under the influence of gravitational force (g-force), substances separate according to their density. Different types of separation are known, including isopycnic, ultrafiltration, density gradient, phase separation, and pelleting.
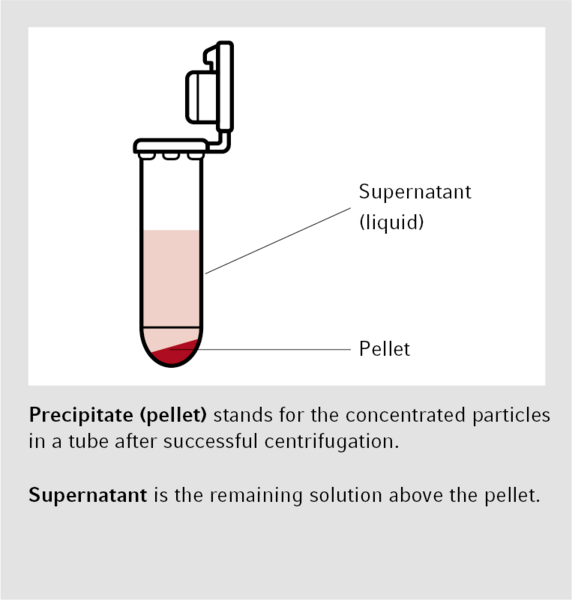
Pelleting is the most common application for centrifuges. Here, particles are concentrated as a pellet at the bottom of the centrifuge tube and separated from the remaining solution, called supernatant. During phase separation, chemicals are converted from a matrix or an aqueous medium to a solvent (for additional chemical or molecular biological analysis). In ultrafiltration, macromolecules are purified, separated, and concentrated by using a membrane. Isopycnic centrifugation is carried out using a ”self-generating” density gradient established through equilibrium sedimentation. This method concentrates the analysis matches with those of the surrounding solution. Protocols for centrifugation typically specify the relative centrifugal force (rcf) and the degree of acceleration in multiples of g (g-force). Working with the rotational speed, such as revolutions per minute (rpm), is rather imprecise.
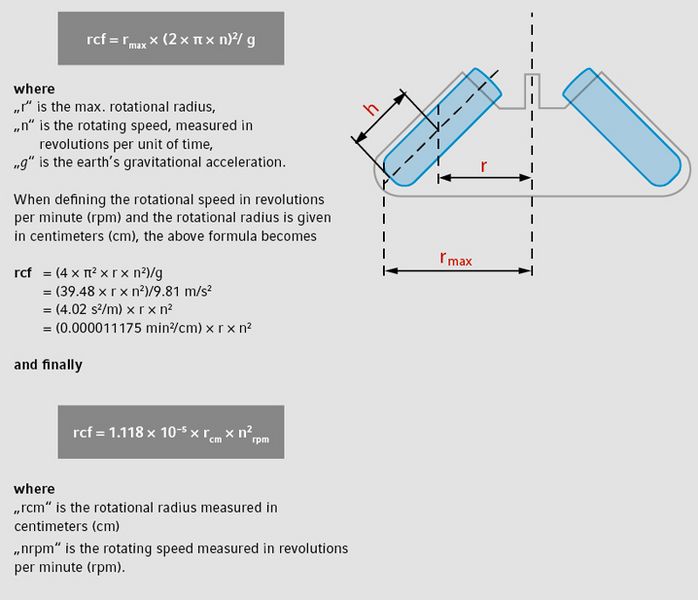
In general, applications for centrifugation specify the degree of acceleration to be applied to the sample rather than specifying a specific rotational speed such as revolutions per minute. The acceleration is typically given in gravity [× g] (or multiples of x g or g-force), the standard acceleration value due to gravity at the Earth’s surface (9.81 m/s2). The distinction between rpm and rcf is important, as two rotors with different diameters running at the same rotational speed (rpm) will result in different accelerations (rcf).
As the motion of the rotor is circular, the acceleration force is calculated as the product of the radius and the square of the angular velocity. Historically known as “relative centrifugal force” (rcf), this is the measurement of the acceleration applied to a sample within a circular movement. This process is measured in units of gravity
(× g).
| Rotor A | Rotor B | ||
|---|---|---|---|
| Speed | 14,000 rpm | 14,000 rpm | |
| Radius | 5.98 cm | 9.50 cm | |
| Gravity | 13,100 × g | 20,817 × g |
*using the formula above
As mentioned, when using
rotors with different radii for centrifugation, the same rcf
(g-force) should be used.
Both centrifuges can spin a rotor with 1.5/ 2 mL tubes at the same speed (14,000 rpm) but the acceleration applied to the samples is very different: 13,100 × g versus 20,817 × g, resulting in different results. To make life easier and to better reproduce the data, some centrifuges have buttons directly on the operating panel for automatic conversion between rpm and rcf. If your centrifuge does not have an rpm-rcf converter, you may use the formula, the rpm-rcf converter found on the homepages of centrifuge suppliers, or a nomogram for conversion. The k-factor is a parameter for the sedimentation distance in a test tube. This factor is also called clearing factor and represents the relative pelleting efficiency of a centrifugation system at maximum rotational speed. In general, the k-factor value is used to estimate the time, t (in hours), required for complete sedimentation of a sample fraction with a known sedimentation coefficient measured in s (svedberg).
A small k-factor represents a more rapid separation. The value of the k-factor is primarily determined by the rotor diameter. Compared to rpm/rcf, the usage of the k-factor has become less important for general centrifugation processes. Especially for ultracentrifugation, the k-factor is still relevant.
If you follow a given protocol, make sure to use the same type of rotor and apply the given relative centrifugal force (rcf) as well as the same temperature and running time. In general, the following major parameters have to be determined for a successful centrifugation run:
A: Type of sample
B: Vessel selection
C: Type of centrifuge
D: Type of rotor
E: Determination of desired relative centrifugal force
F: Defined temperature during centrifugation
The most common rotors in laboratory centrifugation are either fixed-angle or swing-bucket rotors. Only a few applications require special rotors such as continuous-flow rotors, drum rotors, and the like. Flow-through rotors enable continuous flow collection of precipitates. These systems are used, e.g., in harvesting fermenters or for juice production in the food industry. Special customized versions, optimized for the specific application, are necessary.


Fixed-angle rotor
The obvious advantage is the lack of moving parts in the rotor. This results in lower metal stress (longer lifetime), a higher maximum g-force is possible and for many applications, faster centrifugation times can be realized. The limited capacity (less flexibility) of the fixed-angle rotor is the only drawback. The position of the pellet strongly depends on the angle of the tube, it is located from the side to the bottom of the tube when spinning. Most rotors have a 45° angle for the tubes. The larger the angle for the tubes, the tighter the pellet. Smaller rotor angles result in more spread out pellet areas.


Swing-bucket rotor
This kind of rotor is highly flexible for using different tube formats, including SBS-format plates, based on a broad range of adapter systems and a high sample capacity. The moving swing-bucket parts result in increased metal stress for the rotor and the buckets as the bucket weight places a load on the two pivots and grooves. Compared with a fixed-angle rotor, therefore, a swing-bucket rotor is limited to a lower maximum g-force, which leads to longer centrifugation times. Based on the swing-bucket principle, the pellet is located in the bottom of the tube (horizontal position of tube during the run). The recovery by the user is facilitated compared to pellets located at the side of the tube.
Floor-standing centrifuges
Floor-standing centrifuges free up bench space but do need at least one square meter of lab floor space. They are a good choice for high-speed or high-capacity protocols. Among floor-standing centrifuges, choices include ultracentrifuges, super-speed centrifuges, and low-speed centrifuges. An ultracentrifuge is a device for exceptionally high speed. These refrigerated centrifuges have an evacuated chamber to enable a rotational speed of up to 150,000 rpm. The g-force is about 300,000 to 1,000,000 × g. Special vessels that are placed within the rotor or attached to a special rotor are necessary. When g-forces of 40,000 to 60,000 × g are needed, super-speed floor-standing centrifuges are to be used. Low-speed floor-standing devices are generally used for applications like cell culture or blood with less than 10,000 rcf as the maximum g-force.
Bench-top centrifuges
Bench-top centrifuges are available in different sizes:
Microcentrifuges are optimized for low-volume tubes, have a small footprint, and provide 14,000 to 30,000 × g for up to 48 microtubes. Some devices can even be used for a few 15 mL or 50 mL conical tubes or 2 SBS-format plates. Many suppliers offer non-refrigerated and refrigerated versions and different sizes of devices based on their tube capacity.
Offering a bigger rotor chamber, multipurpose centrifuges allow a broad range of rotors to be used (highly versatile). In addition to a flexible rotor system, specific adapter systems enable use of a wide variety of different kinds of tubes and bottles (from 0.2 mL to 1,000 mL) as well as plates. The maximum speed heavily depends on the vessel’s characteristics.
LabTeam Scandinavia AB
Vasatorpsvägen 1G
Godsmottagning – LabTeamet
254 57 Helsingborg
Sweden
LEASING / HYRA / AVBETALNING
Vårt samarbete med Wasa Kredit gör att du snabbt och enkelt får förslag på finansiering som passar dina behov Finansiering upp till 100%. Ni slipper binda ert kapital.
ANMÄL DIG TILL VÅRT NYHETSBREV
Din epost (obligatorisk)
För att ge de bästa upplevelserna använder vi teknik som cookies för att lagra och/eller komma åt enhetsinformation. Genom att godkänna dessa tekniker kan vi behandla data som surfbeteende eller unika ID:n på denna webbplats. Att inte samtycka eller återkalla samtycke kan påverka vissa funktioner och funktioner negativt.
Har du lagt till produkter till ”Mina produkter” så glöm inte skicka iväg din pris- eller produktförfrågan också. Skapa gärna ett konto så sparas alla dina förfrågningarna under ”Mitt konto” sidan.