Vattenegenskaper
Vattenegenskaper
Vatten är livsviktigt för livet på vår planet, och täcker ungefär 70% av dess yta. Det är en viktig komponent i levande organismer och spelar en betydande roll i att forma miljön, klimatet och vädret. Dess enastående egenskaper gör det till en väsentlig del av vår existens.
042-300 91 30
Frågor? Ring kundtjänst
Hydrolab – Water properties
Water is one of the most important and most common chemical compounds on our planet. It was thanks to her that it was possible for life to arise and exist. Its universality is impressive, it covers about 70% of the Earth’s surface. It is also an important component of living organisms, the atmosphere or the lithosphere. It has a huge impact on shaping nature, climate and weather phenomena. Water is a necessary chemical compound for our existence. Why these extraordinary properties?
They result from its structure as well as physical and chemical properties. A water molecule consists of two hydrogen atoms connected by one oxygen atom. Is a non-linear molecule, the angle between polarized, covalent hydrogen-oxygen-hydrogen bonds is 104.50. Its three-dimensional shape is a tetrahedral system, which in addition to two hydrogen atoms also includes two electron pairs at the oxygen atom. Due to this structure, the molecule behaves like an electric dipole, it is strongly polar, which gives excellent conditions for hydrogen bonds. They occur in all states. This is an association phenomenon of attracting one water molecule to the negative pole and the positive pole to the other. This allows, for example, ice, which is a solid, to be lighter than its liquid variety.
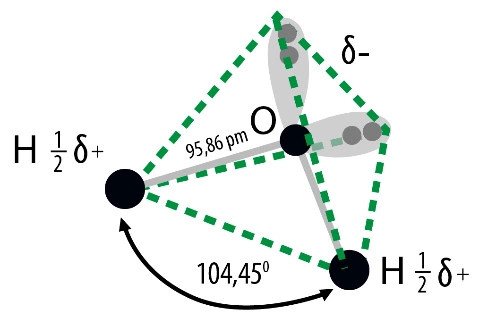
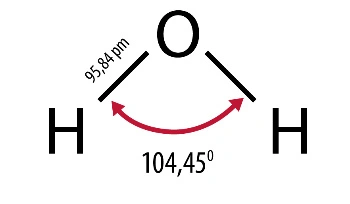
Water is also an important, often underestimated, role in any well-functioning laboratory. The correctness of test results and conclusions as well as the correctness of analytical apparatus depend on its purity. Designing a new laboratory should start with planning the needs for purified water in the right quantities and quality.






